Ellipsys.
A minimally invasive approach to AV fistula creation.
A minimally invasive approach to AV fistula creation.
The Ellipsys® Vascular Access System has received FDA approval in the U.S. and European CE Mark approval for the creation of an arteriovenous fistula in patients with chronic kidney disease requiring hemodialysis.
Three strong reasons to rely on Ellipsys:
1.
Its reputation as the only system of its kind with published U.S. data, cleared for use in HOPD, ASC and OBL settings.
2.
Its patented tissue fusion technology, enabling immediate, minimally invasive creation of permanently fused anastomosis.
3.
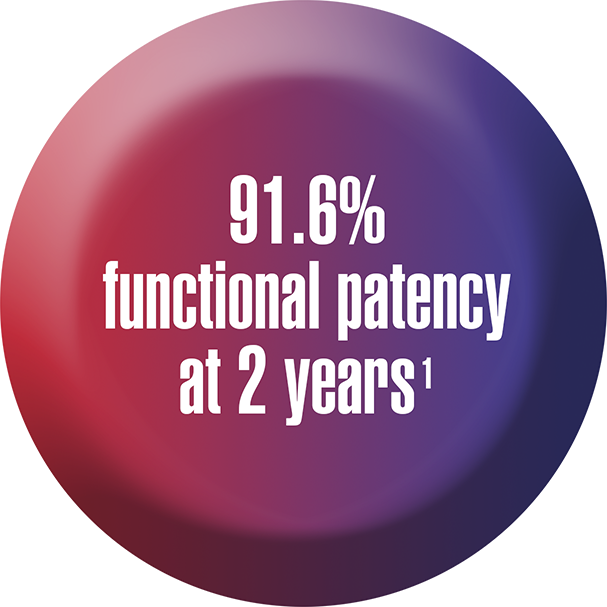
Its track record of achievement in over 2000 procedures, with long-term functional patency. And that’s reason enough to learn more about Ellipsys today.